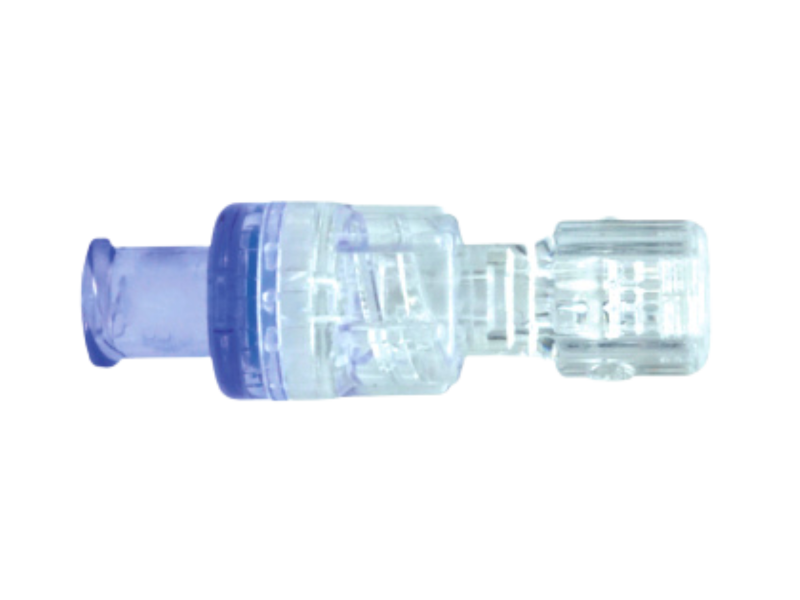
Auxiliary Water Jet Connector for Olympus® & Ambu® Endoscopes
Auxiliary Water Jet Connector for Olympus® & Ambu® Endoscopes
Product Code: G-CN0101
- 1-2 Days Express Delivery
- Box of 100
- Sterile
- Latex Free
- Olympus®, Ambu® Endoscopes
- Colonoscopy, Gastroscopy
Couldn't load pickup availability
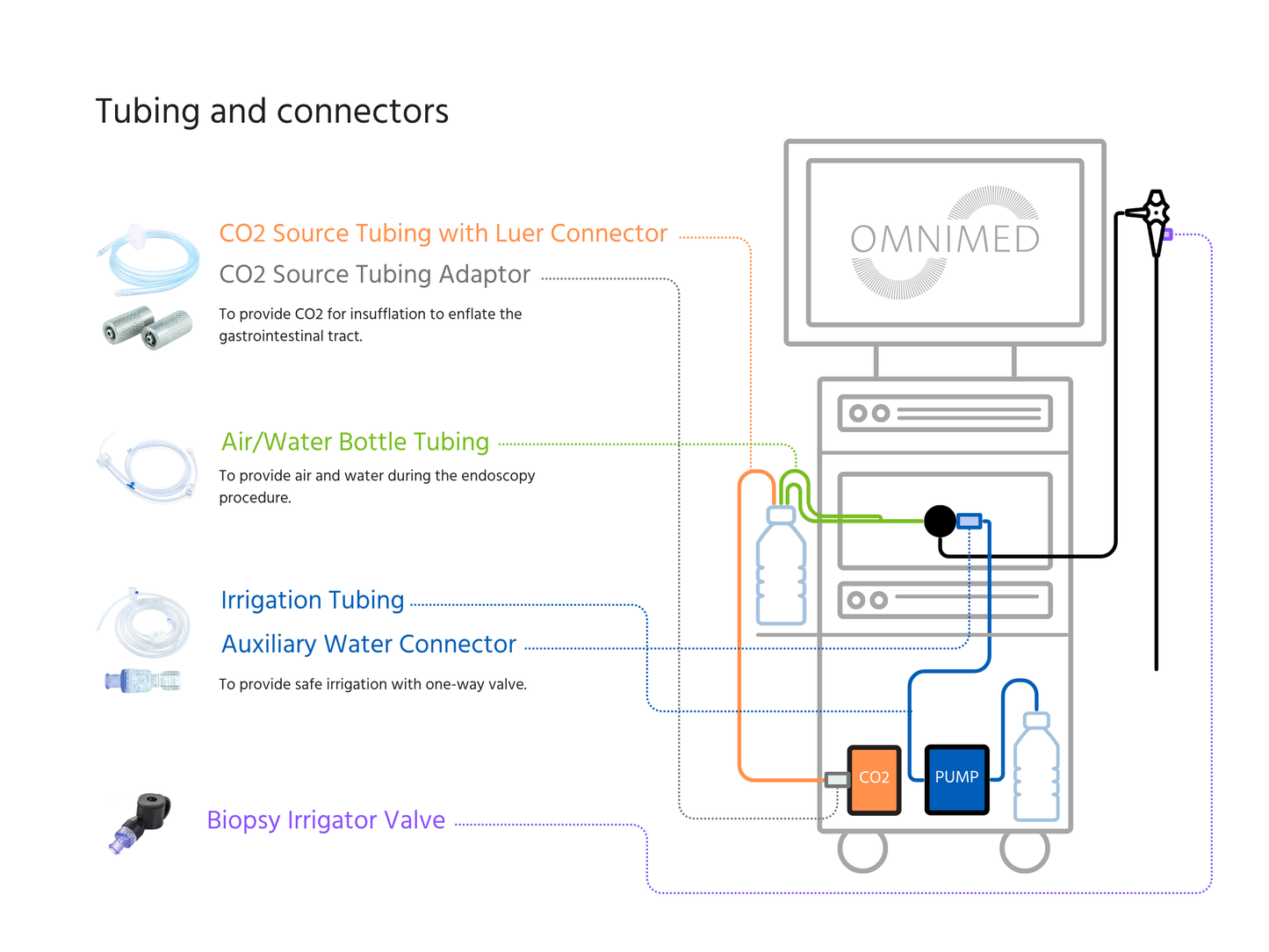
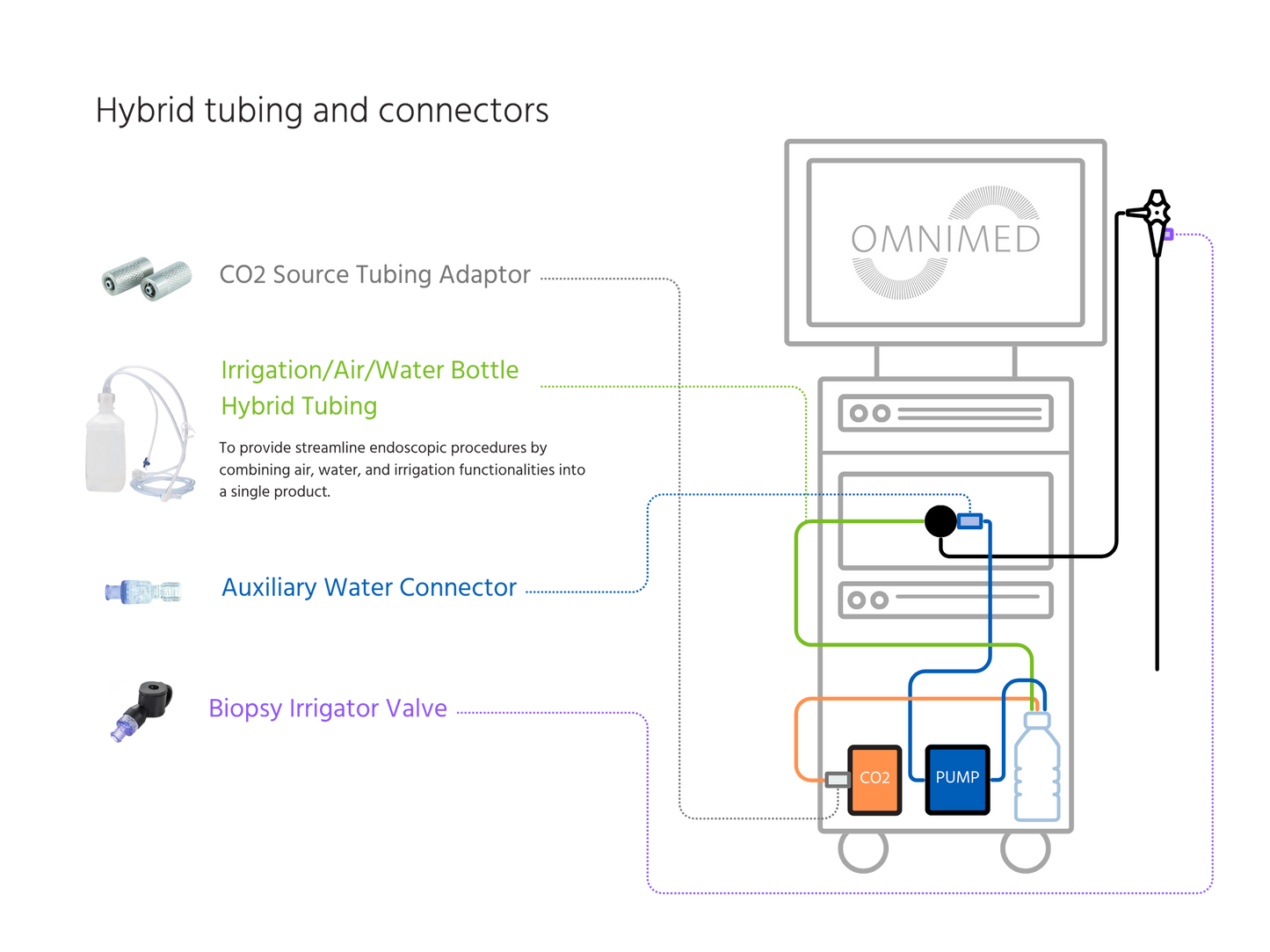
Compatible with Olympus® & Ambu® endoscopy systems, this sterile auxiliary water jet connector ensures a tight, reliable seal during gastroscopy and colonoscopy procedures. It allows seamless connection to irrigation systems for consistent flushing and optimal visibility throughout the procedure.
Individually wrapped and single-use, it helps endoscopy teams maintain best practices in infection prevention and workflow efficiency.
Compatible with:
Order Direct
Order Direct
Order directly from Omnimed for benefits including prior quotations, dedicated sales support, and product demos. Please provide an official purchase order number, delivery address, and contact details for your direct orders. If you have NHS purchase orders, kindly send them to sales@omnimed.co.uk from your NHS email account.
Order direct with code G-CN0101
National Frameworks Agreements
National Frameworks Agreements
We are proud to work with the NHS. Many of our products are available via the NHS Supply Chain (England), HSC Business Services Organisation (Northern Ireland), NHSS National Procurement (Scotland), and GIG Cymru/NHS Wales Shared Services Partnership (Wales).
Eco Bundle and Green Credentials
Eco Bundle and Green Credentials
We take pride in our eco-friendly initiatives as we progress towards our Green Endoscopy mission.
This product is available as part of an Eco Bundle. These bundles are more time-efficient for purchasing, more environmentally friendly in terms of shipping and much more cost-effective as you are only paying for one set of shipping fees. Contact us to order your Eco Bundle today!
Auxiliary Water Jet Connector for Olympus® & Ambu® Endoscopes also benefits from the following eco-credentials:
.
Electronic Instructions for Use
Electronic Instructions for Use
In line with our dedication to ISO standards and a paperless office environment, and measures to support environmental sustainability, we have made the eIFU available for download.
This eIFU is coming soon.
Electronic Safety Data Sheet
Electronic Safety Data Sheet
Where available, the Safety Data Sheet will be downloadable here.
There is no SDS available for this product at this time.
ISO Accreditation & Product Feedback Survey
ISO Accreditation & Product Feedback Survey
Omnimed complies with the requirements of ISO 13485:2016 [Medical devices | Quality management systems | Requirements for regulatory purposes]. It specifies requirements for a quality management system where an organisation needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Such organisations can be involved in one or more stages of the life cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device, and design and development or provision of associated activities
As part of our continuous commitment to quality and our ISO accreditation, we value our customers' feedback. We kindly ask that you take a moment to complete our product experience and evaluation surveys to help us continually improve our products and services. We greatly appreciate your participation.
Product Experience survey (1 min)Product Evaluation survey (5 mins)
Best selling products
Expore our product range and find the perfect solutions to meet your needs.